
Blog Post
From the unbridled mind of Jim Boyd, DDS to the operatories of thousands and thousands of dentists around the world, more than 1 million NTIs have successfully been delivered to patients.
Many dentists inform their patients they are getting an NTI to protect their teeth, prevent migraines, or treat headaches. But something else happens. Instead of delivering a device that could positively change their patients’ lives, some of your colleagues mistakenly provide devices that may worsen symptoms or create new adverse conditions.
Not all splints are created equal.
Allow me to explain.
Anterior Programmer ≠ NTI
An anterior deprogrammer is not an NTI, but an NTI is an enhanced anterior deprogrammer.
Read that last sentence again.
Anterior deprogrammers have been utilized by dentists for decades. The concept of the anterior deprogrammer involves creating a small, single contact point for the incisors to meet, while disengaging all the posterior teeth. A simple way to put it according to Raj Updadya, DMD is that it “clears the dance floor” of any noxious posterior interferences.1
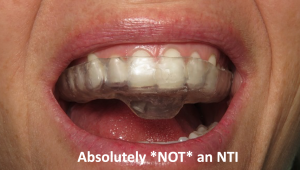
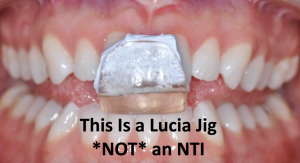
This muscle deprogramming reduces or relaxes muscle activity levels to eliminate muscle pain, tension, or discomfort.2 Some anterior deprogrammers such as Lucia jigs or Pankey anterior bite stops are fitted chairside and worn only on the anterior teeth. Because of the partial coverage, some clinicians mistakenly refer to these types of anterior deprogrammers as “NTI appliances.
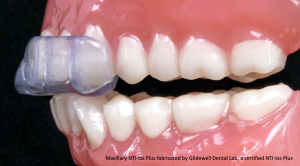
This fallacy isn’t just a matter of semantics. It’s like comparing apples to aardvarks. The standard NTI-tss Plus appliance extends coverage to the canines. It is custom-fabricated by an NTI-certified laboratory and can cover additional teeth on either arch as specified by the prescribing dentist.
That’s only where the differences begin.
The most significant difference between an anterior deprogrammer and the NTI-tss Plus is in functionality and indications for use.
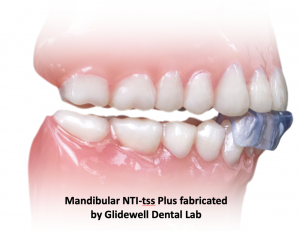
The NTI-tss Plus FDA clearance states that it is “A device to be used in the prophylactic treatment of medically diagnosed migraine pain as well as migraine associated tension-type headaches, by reducing their signs and symptoms through reduction of trigeminally innervated muscular activity, and; for the prevention of bruxism and TMJ syndrome through reduction of trigeminally innervated muscular activity.”3 In simple terms, the NTI-tss Plus is FDA cleared for the treatment of migraines, tension-type headaches, and TMJ disorder due to clenching and grinding. The bag of blanks you’ve bought can’t make that claim, nor can it deliver those results.
Anterior deprogrammer designs vary, but all of them allow canine contact in lateral excursions. Some appliances even provide it in a centric clench because the anterior point stop is that wide.
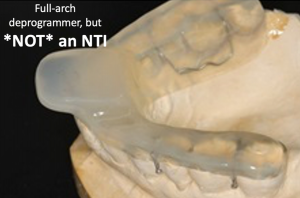
While canines are anterior teeth, they function as posterior teeth. Hattori, et al showed that biting forces were greatest with molars in occlusion and forces were reduced as teeth were removed from occlusion moving toward the mesial; less teeth in occlusion = less biting forces.
This leads us to the enhancement – the key differentiator – the proprietary “magic” of the NTI-tss Plus. Its patented Discluding Element ensures that canines and all posterior teeth are out of occlusion in a centric clench and all excursive movements. This disclusion has been shown to reduce clenching intensity by ~70% which allows muscles to relax while also reducing nociception.
The NTI therapeutic protocol instructs clinicians to:4
It’s paramount that clinicians follow these steps when seating NTI appliances. Too often, well- intentioned clinicians prescribe knock-off NTIs. Labs that are not NTI-certified lack the training to properly fabricate NTI devices, appropriately address clinician questions, and we’ve seen cases in which this has resulted in severely negative outcomes for patients.
Be confident in your NTI-tss Plus devices and deliver the best results for your patients.